Garnet-based batteries to power devices and cars
Charging their devices is one of the basic concerns of every individual. Recently, a group of researchers at the University of Calgary, Canada, has designed a garnet-based, button-sized rechargeable battery, which can help in powering electronics, vehicles, and grids for storage of renewable energy. The novel battery made by Venkataraman Thangadurai and team is a non-flammable, chemically steady, and able to function safely at an elevated voltage than the batteries available at present.
Electric vehicles have the potential to modernize the transportation but they require better-performing and safer batteries. The existing lithium-ion batteries have various problems such as flammability poor chemical stability, leakage, and limited energy density or operating voltage. This new next-generation battery has several potential uses, including in solid-state gas sensors, consumer electronics, electric vehicles, and electrical grids for accumulating power produced by renewable energy and offering electricity at the time of peak requirements.
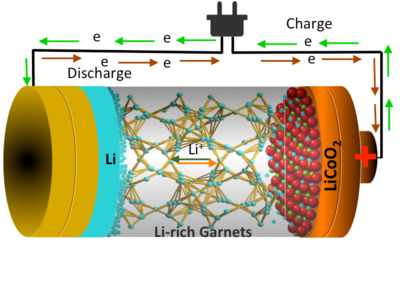
The available lithium-ion batteries utilized in plug-in hybrid vehicles, electric vehicles, and portable electronics utilizes lithium salts and membranes of organic polymers as the electrolyte. The electrolyte present in the battery divides 2 electrodes and then mediates the lithium ions between the electrodes throughout discharging and charging cycles. The organic polymer-based electrolytes used at present are combustible creating a safety problem.
In the present study, the scientists have used a solid ceramic electrolyte for their batteries, which is inflammable, rather than the organic polymers. The team has also used a method, known as atomic layer deposition, to lay a slim film of aluminum oxide on the garnet framework covering the ceramic electrolyte.
Thus, the team was successful in demonstrating a negligible interface resistance among the ceramic electrolyte and the lithium metal anode interface with the garnet-based, chemically engineered, and ceramic electrolyte. This ultimately led to the rapid transfer of charges and high overall performance. Isn’t it great?